Ansella Therapeutics is developing a range of treatment that focus on Women’s Health including Menopause, Vaginal mucositis, Endometriosis, Uterine Fibroids and vaginal Infection. Vaginal formulations leverage the Biomyme V platform. The initial pipeline focuses on vaginal dryness, dyspareunia and radio/chemo induced vaginal mucositis.
Our product pipeline
| PRODUCT | FORMULATION | INDICATION | STATUS |
|---|---|---|---|
| CERYNE | Biomyme V | Vaginal Dryness |  |
| ATX-001 | Biomyme V | Vaginal Dryness, Dyspareunia |  |
| ATX-002 | Biomyme V Estradiol 0.009% | Dyspareunia, Atrophic Vaginitis |  |
| ATX-003 | Biomyme V, Hyaluronic Acid | Radiotherapy or chemotherapy induced vaginal mucositis |  |


Cerynë Intimate Care
Product
Cerynë Intimate Care
Formulation
Biomyme V
Indication
Vaginal Dryness
Website
Cerynë Intimate Care is the world’s first biomimetic formulation which works with your body to restore comfort and balance.
Cerynë Intimate care has been launched both directly to consumers and through physician channels as an intimate care product for vaginal dryness. The product has been develop for women with everyday vaginal dryness and designed for vulvovaginal application.
- Restores vaginal moisture, and lubricates
- Soothes and nourishes delicate intimate tissues
- Provides long lasting comfort
- Formulated to support a healthy vaginal microbiome
- No mess formulation avoids embarrassing leakage
- Safe and gentle for everyday use


ATX-001
Formulation
Biomyme V
Indication
Vaginal Dryness, Dyspareunia
ATX-001 is a non-hormonal, non-prescription Biomyme V platform silicone-based cream designed to restore vaginal pH, replenish essential vaginal biochemistry, provide lubrication, and moisturize the vagina in peri- and post-menopausal women.
ATX-001 is a Class II medical device with Non-Significant Risk (NSR) that will proceed along the 510(k) regulatory pathway.
Future clinical studies will specifically look at the use in different aspects of VVA such as dyspareunia and microbiome dysfunction.
Additionally, there may be supportive indications in Graft Versus Host Disease and vulval lichen planus.

ATX-002
Formulation
Biomyme V Estradiol 0.009%
Indication
Dyspareunia, Atrophic Vaginitis
ATX-002 is expected to enable the lowest estradiol dose on the market – <0.01%
Using the Biomyme V platform will enhance the delivery and local efficacy of local estrogen-based therapy and allow a lower dose of estradiol to delivery improved efficacy and lower side effects than current marketed products. We believe that reductions in osmotic epithelial cell damage will contribute to improved efficacy, onset of action and lower side effects.
ATX-002 will follow a 505(b)(2) regulatory pathway, for dyspareunia and atrophic vaginitis and provide fast access to market.
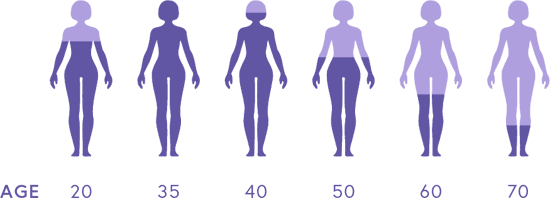
Estrogen Levels


ATX-003
Formulation
Biomyme V, Hyaluronic Acid
Indication
Radio/chemo therapy induced vaginal mucositis
ATX-003 Vaginal Cream is a class IIb medical device, that leverages the Biomyme V platform to deliver hyaluronic acid/cholesterol complex
Chemotherapy can damage the vaginal tissue, which can lead to ulcerations or disruption in the integrity of the mucosa that may cause mucositis, vaginal irritation and increase the risk of infection.
The vaginal and vulval epithelium are highly sensitive to the effects of pelvic irradiation or pelvic radiotherapy. Radiotherapy can damage vaginal epithelium, connective tissues and small blood vessels, causing inflammation and cell death. The subsequent reduced blood supply, tissue hypoxia, loss of elastin and collagen deposition leads to thinning of the vaginal mucosa, loss of lubrication, scarring and fibrosis.
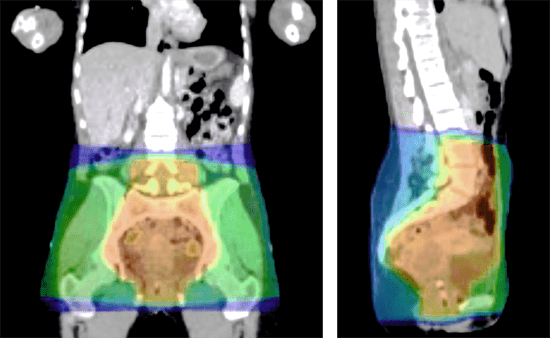
ATX-003 forms a protective layer over the exposed nerve endings and the mucosa, helps relieve symptoms of neurological injury caused by vulvovaginitis and aids the healing process. The specially designed applicator helps to ensure the entire affected area can be reached.